For the redox reaction MnO2 + C202 +H- Mn2+ + CO2 + H2O the correct coefficients of the reactants the balanced equation are MnO4 C204 H+ (1) 16 5 (2) 2 5 16 (3) 2 (4) 5

Influence of Common Anions on the Coordination of Metal Cations in Polyalcohols - Teichert - 2019 - European Journal of Inorganic Chemistry - Wiley Online Library

Rare and Nonexistent Nitrosyls: Periodic Trends and Relativistic Effects in Ruthenium and Osmium Porphyrin-Based {MNO}7 Complexes | ACS Omega
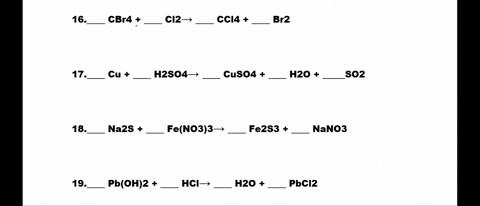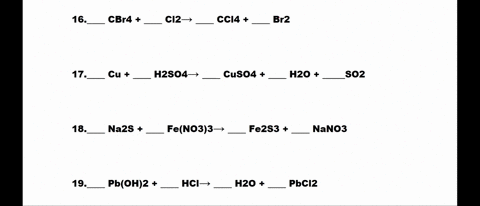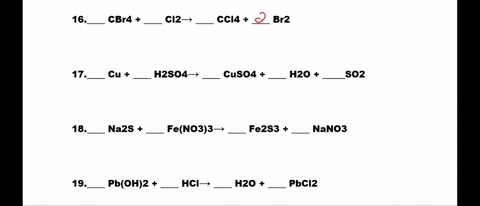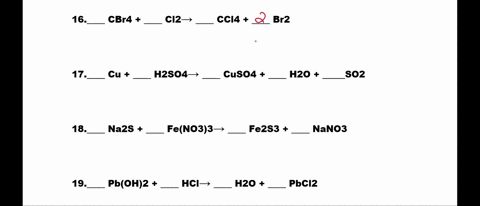
SOLVED: 1. N2 + H2 â†' NH3 2. CH4 + O2 â†' CO2 + H2O 3. P4 + O2 â†' P2O3 4. H2 + NO â†' N2 + H2O 5. Na +

Bac sciences - ✓💡⚠️بعض المزدوجات الهامة تفاعل أكسدة اختزال المستعملة بالسنة الختامية.⚠️ PC&SM&SVT(2bac)✓ #فيزياء_و_كيمياء | Facebook

Crystal structures of various high-pressure metal (Me=Mn 2+ , Co 2+ ,... | Download Scientific Diagram

MnO_(4)^(-)` oxidises `H_(2)O_(2)` to `O_(2)` in acidic medium `xMnO_(4)^(-)+yH_(2)O_(2)+zH^(+) rarr Mn^(2+)+O_(2)+H_(2)O` Coefficients `x`, `y` and - Sarthaks eConnect | Largest Online Education Community

Preparation of Manganese Oxide Nanoparticles with Enhanced Capacitive Properties Utilizing Gel Formation Method | ACS Omega

Preparation of Manganese Oxide Nanoparticles with Enhanced Capacitive Properties Utilizing Gel Formation Method | ACS Omega

For the redox reaction MnO2 + C202 +H- Mn2+ + CO2 + H2O the correct coefficients of the reactants the balanced equation are MnO4 C204 H+ (1) 16 5 (2) 2 5 16 (3) 2 (4) 5

Processes | Free Full-Text | A Sustainable Process to Produce Manganese and Its Alloys through Hydrogen and Aluminothermic Reduction