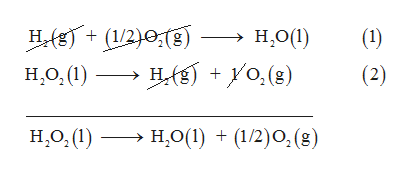Comment équilibrer : H2O2 → O2 + H2O (peroxyde d'hydrogène, dioxygène, eau) | Physique-Chimie - YouTube

2H202 alkaline medium *2H20 + 02 the proposed mechanism is as given below : (1) H2O2 +1 → H2O+IO (slow) (2) H202 + 10 + H20+1+02 (fast) (i) Write rate law the
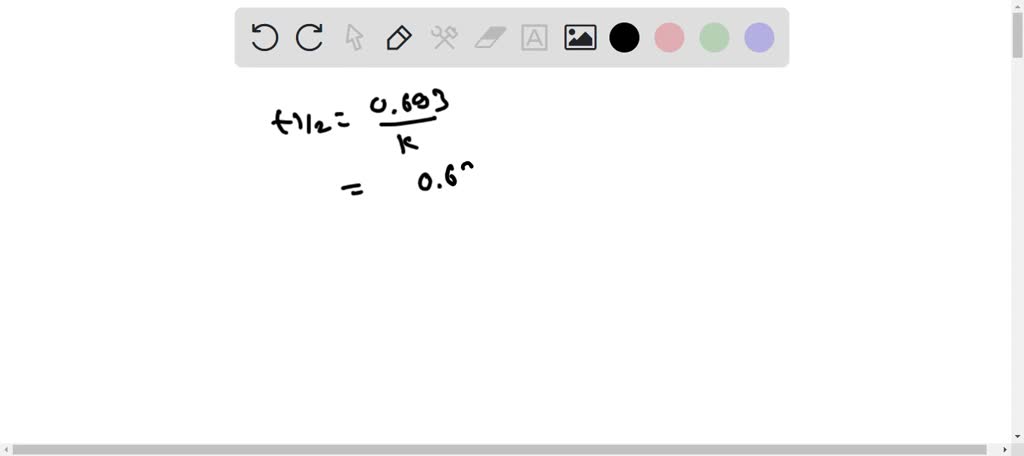
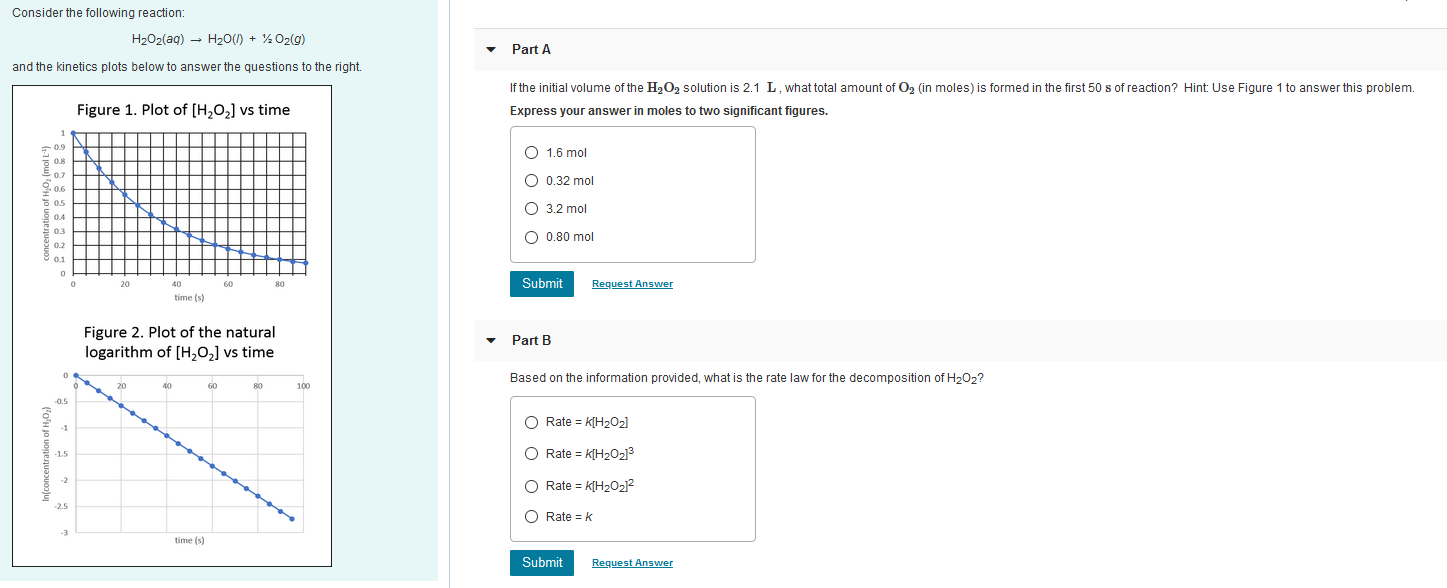
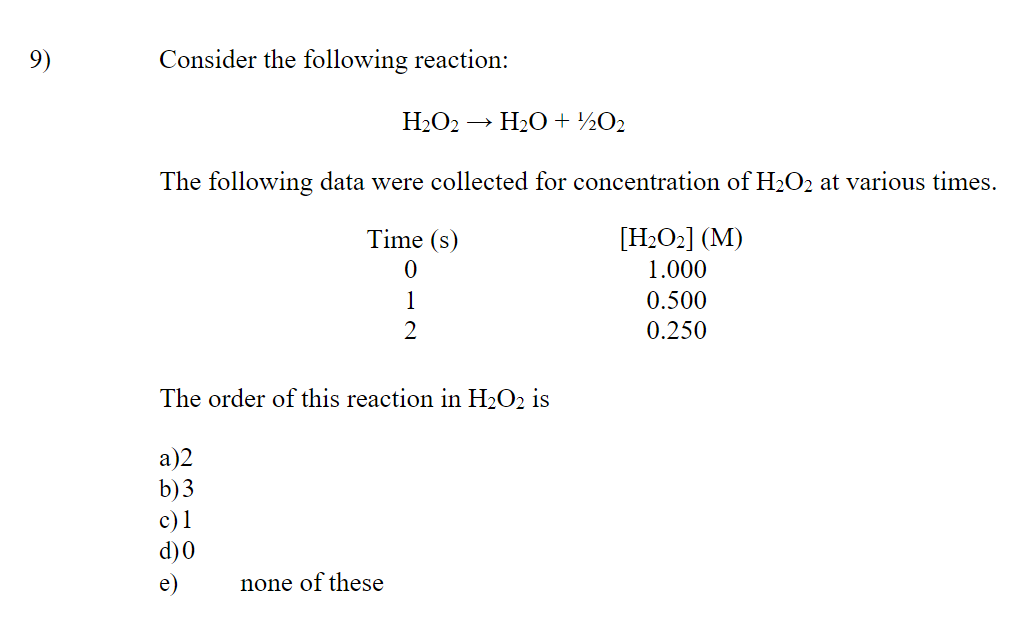
SOLVED: H2O2(aq) ⟶ H2O(l) + 1/2O2(g) a) The above chemical equation is for a first-order reaction. At 303 K, the rate constant equals 9.4 × 10^(-4) s^(-1). Calculate the half-life at this
I) H2O2 + O3 → H2O + 2O2 (II) H2O2 + Ag2O → 2Ag + H2O + O2 Role of hydrogen peroxide in the - Sarthaks eConnect | Largest Online Education Community
![SOLVED: In the reaction H2O2(aq) â†' H2O(l) + 1/2 O2(g), the initial concentration of H2O2 is 0.2546 M, and the initial rate of reaction is 9.32×10^(-4) M/s. What will be [H2O2] at SOLVED: In the reaction H2O2(aq) â†' H2O(l) + 1/2 O2(g), the initial concentration of H2O2 is 0.2546 M, and the initial rate of reaction is 9.32×10^(-4) M/s. What will be [H2O2] at](https://cdn.numerade.com/ask_previews/68c50aa-0834-010e-1c31-cf1f024cbc64_large.jpg)
SOLVED: In the reaction H2O2(aq) â†' H2O(l) + 1/2 O2(g), the initial concentration of H2O2 is 0.2546 M, and the initial rate of reaction is 9.32×10^(-4) M/s. What will be [H2O2] at
Free Online Help: Given the following delta H values H2+1/2O2--->H2O delta H =-285.8 H2O2---->H2+O2 delta H = 187.6 Calculate delta H rxn for the following reaction H2O2--->H2O + 1/2O2

16.38d | Would hydrogen peroxide be a suitable candidate for fuels: H2O2(l) → H2O(g) + 1/2O2(g) - YouTube







![Odia] H2O2 to H2O + 1/2 O2 is order reaction. Odia] H2O2 to H2O + 1/2 O2 is order reaction.](https://static.doubtnut.com/ss/web-overlay-thumb/11729098.webp)