
Fabricating collagen films with oxygen-release capabilities: 1,7-octadiene PECVD encapsulation of calcium peroxide - American Chemical Society

Identify the substance oxidized, substance reduced, oxidizing agent and reducing agent in the following: 1 Cl2 + 2NaBr 2NaCl + - Science - Chemical Reactions and Equations - 13638355 | Meritnation.com

SOLVED: Consider the combustion of Ca. What are the expected products of the reaction? Select all that apply: CaO2 CaZo H2O CaO CO2
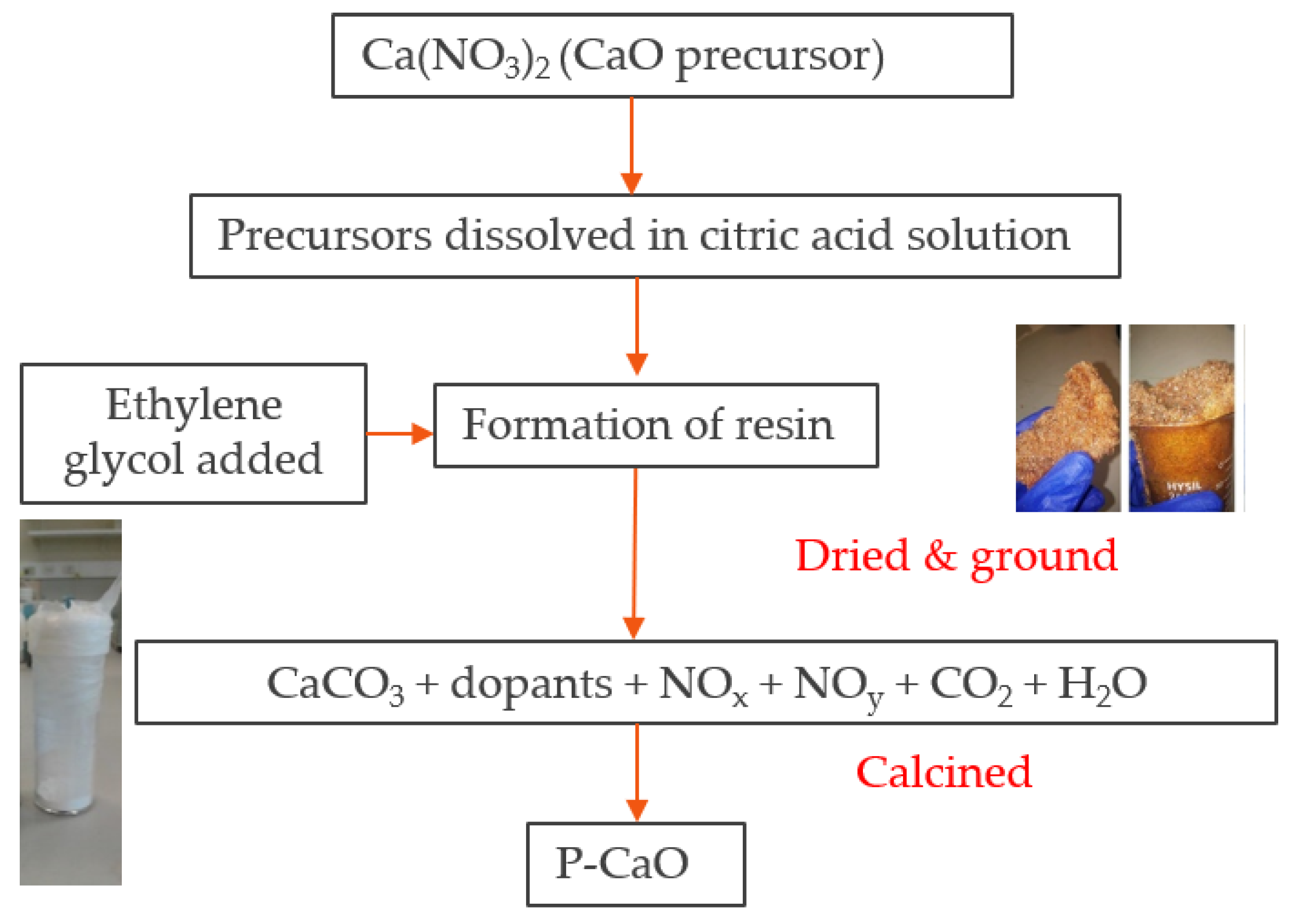
Applied Sciences | Free Full-Text | Comparative Kinetic Analysis of CaCO3/CaO Reaction System for Energy Storage and Carbon Capture
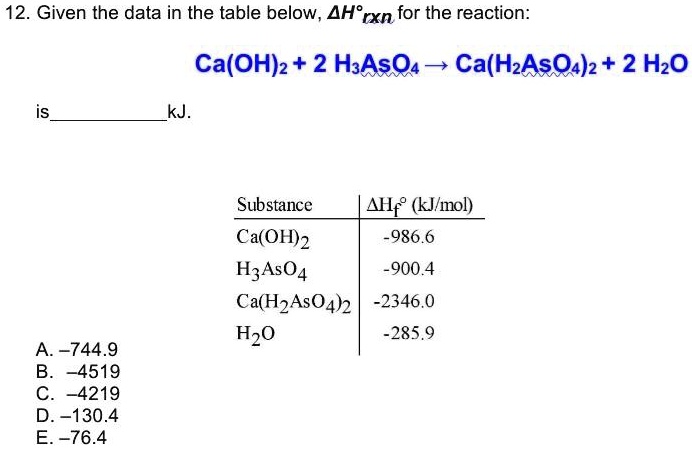
SOLVED: Given the data in the table below, calculate the enthalpy change (ΔH) for the reaction: Ca(OH)2 + 2 H3AsO4 â†' Ca(H2AsO4)2 + 2 H2O Substance ΔH (kJ/mol) Ca(OH)2 986.6 H3AsO4 900.4

Influence of calcium peroxide on fermentation pattern and protozoa in the rumen: Archiv für Tierernaehrung: Vol 32, No 7-8

Polymers | Free Full-Text | Synthesis of Controlled-Release Calcium Peroxide Nanoparticles Coated with Dextran for Removal of Doxycycline from Aqueous System

Please help with all parts and show work. The decomposition of Ca(OH)_2(s) into CaO(s) and H_2O(g) at constant pressure requires the addition of 109 kj of heat per mole of Ca(OH)_2. (a)








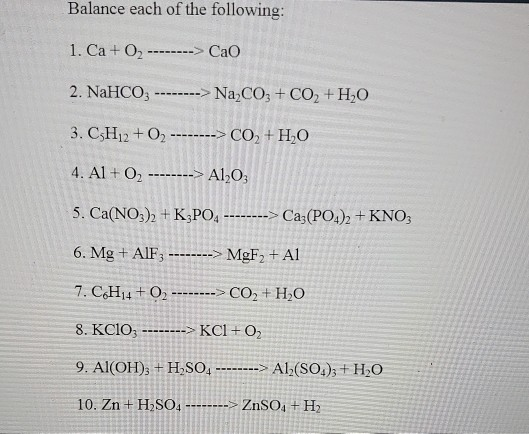
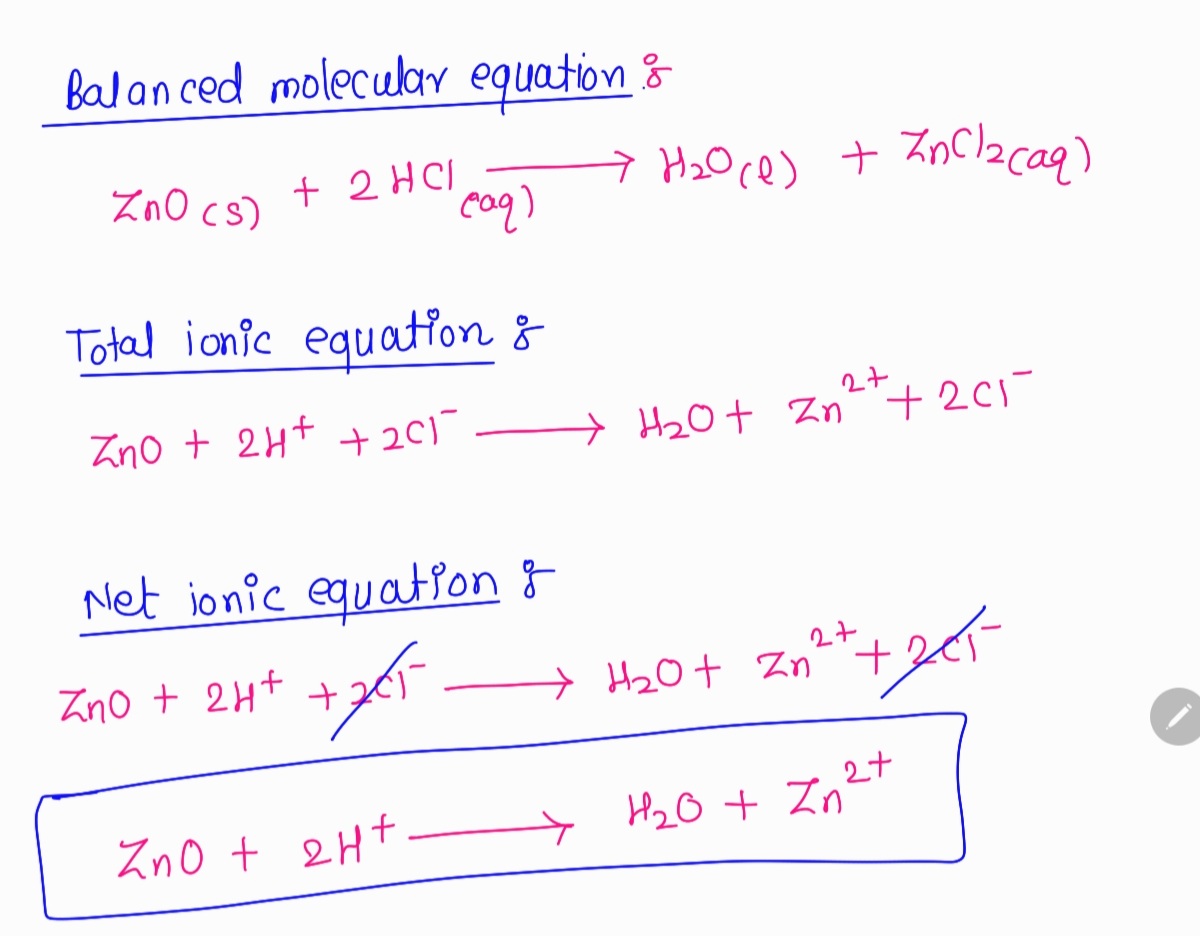
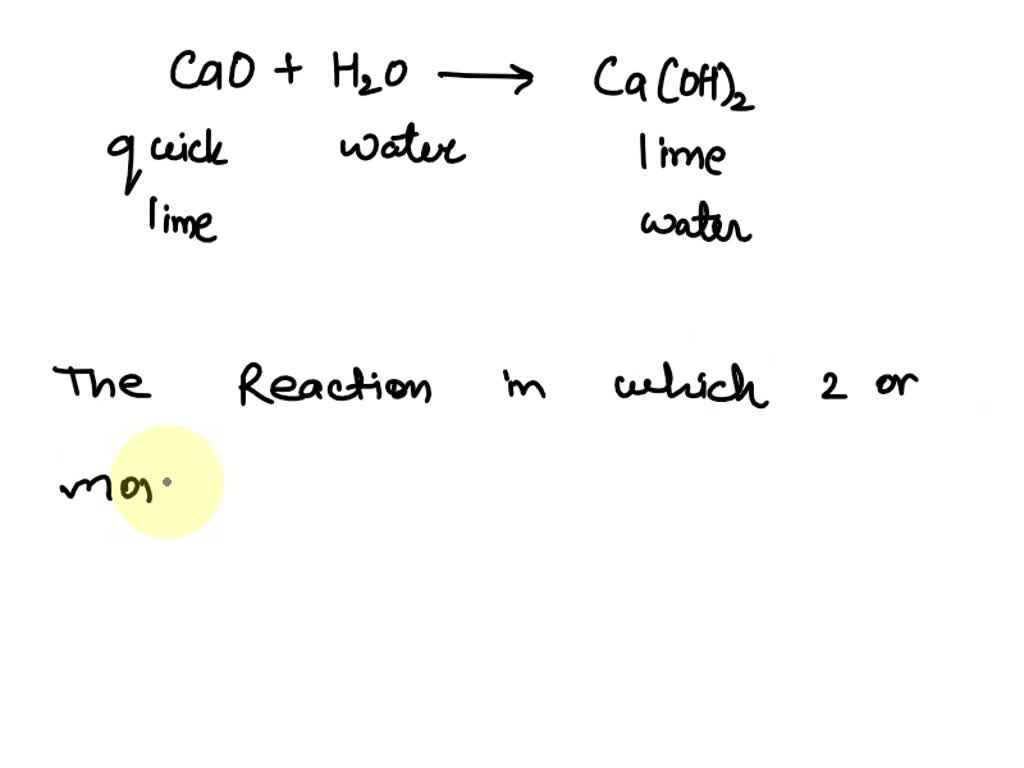
![Punjabi] What type of reactions are represented by following equation Punjabi] What type of reactions are represented by following equation](https://static.doubtnut.com/ss/web-overlay-thumb/10335489.webp)



