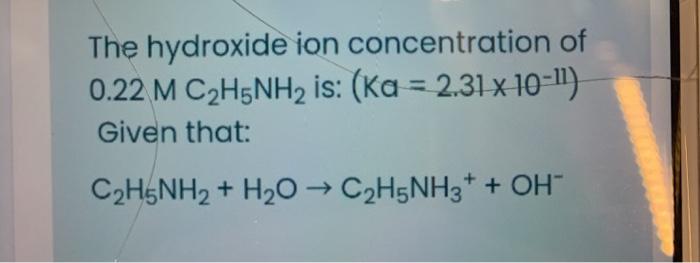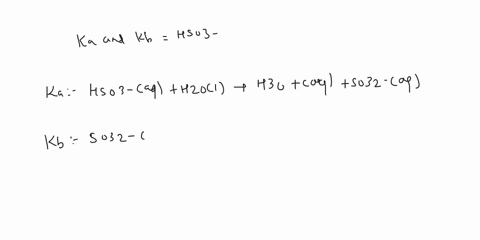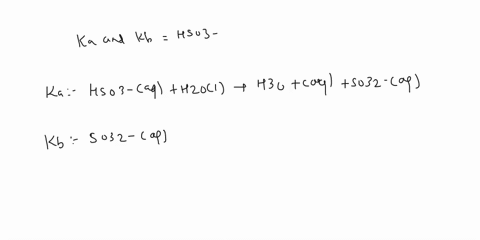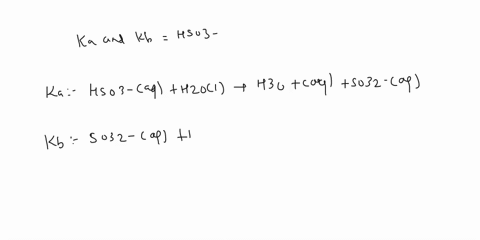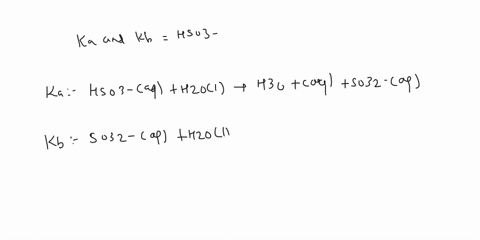
SOLVED: a.) CO3^2- + H2O -> HCO3^- + OH- b.) C6H5NH2 + H2O -> C6H5NH3^+ + OH- c.) C2H5NH2 + H2O -> C2H5NH3^+ + OH-
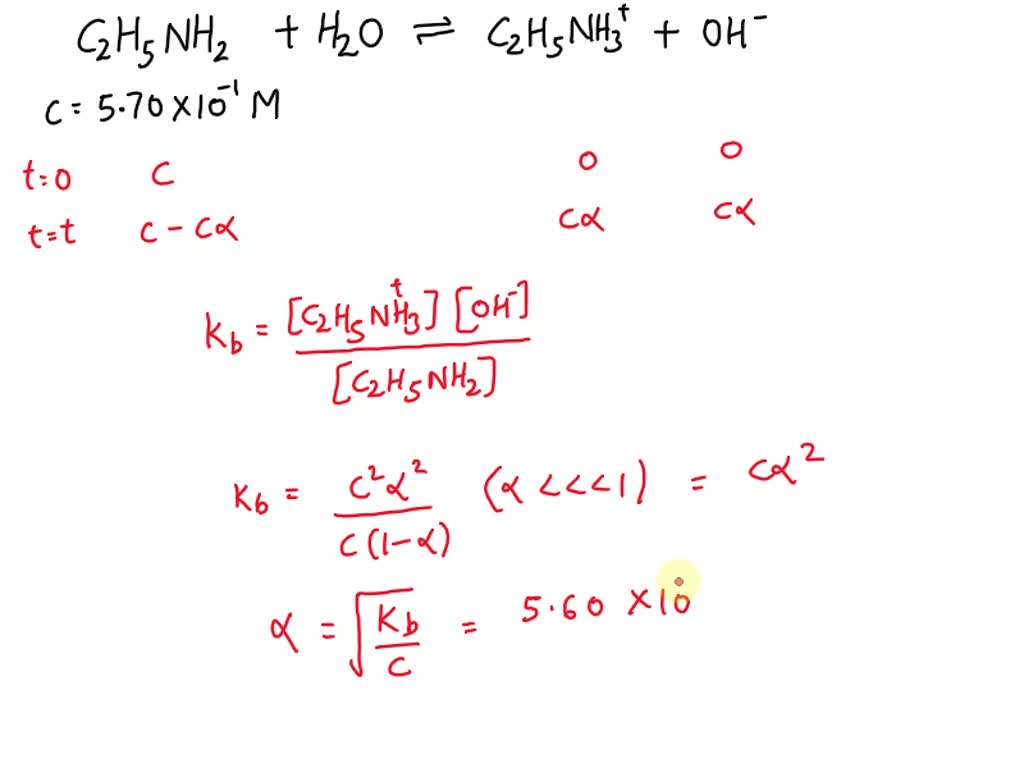
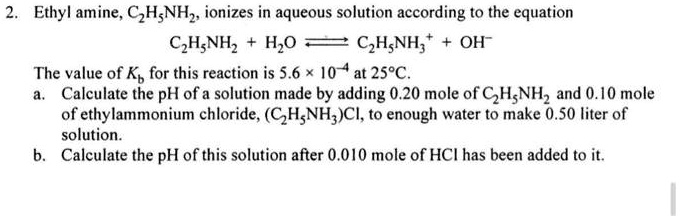
SOLVED: Calculate the pH of a 5.70×10^-1 M aqueous solution of ethylamine hydrochloride (C2H5NH3Cl). (For ethylamine, C2H5NH2, Kb = 5.60×10^-4.)
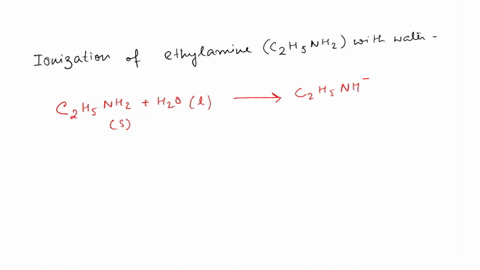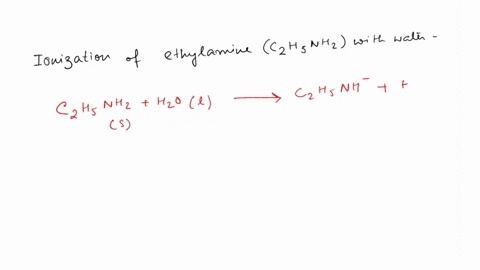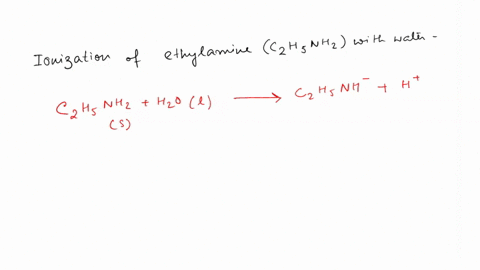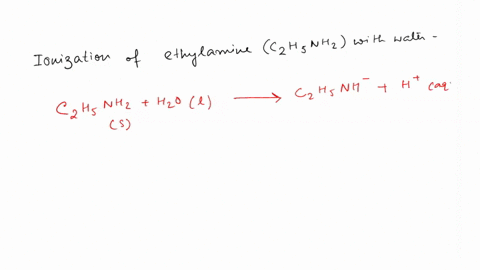
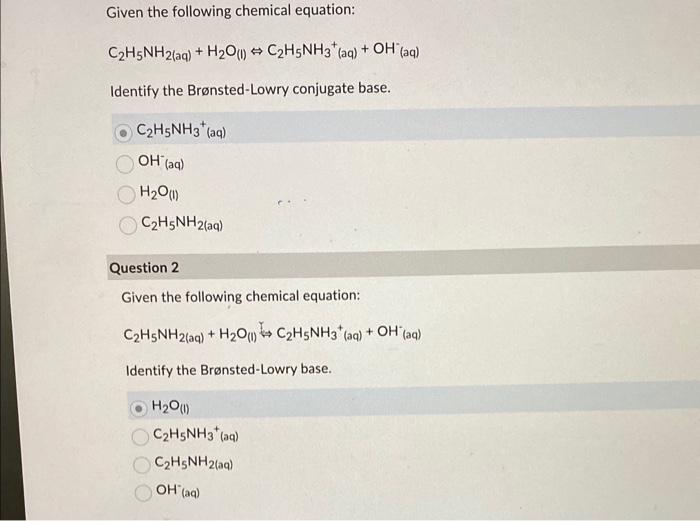
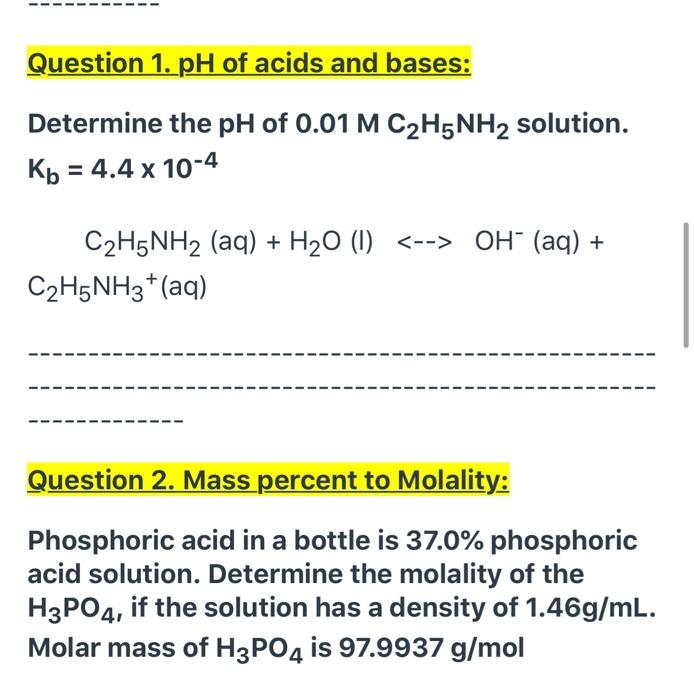
SOLVED: Write the equation for the ionization of ethylamine (C2H5NH2), a weak molecular base, with water
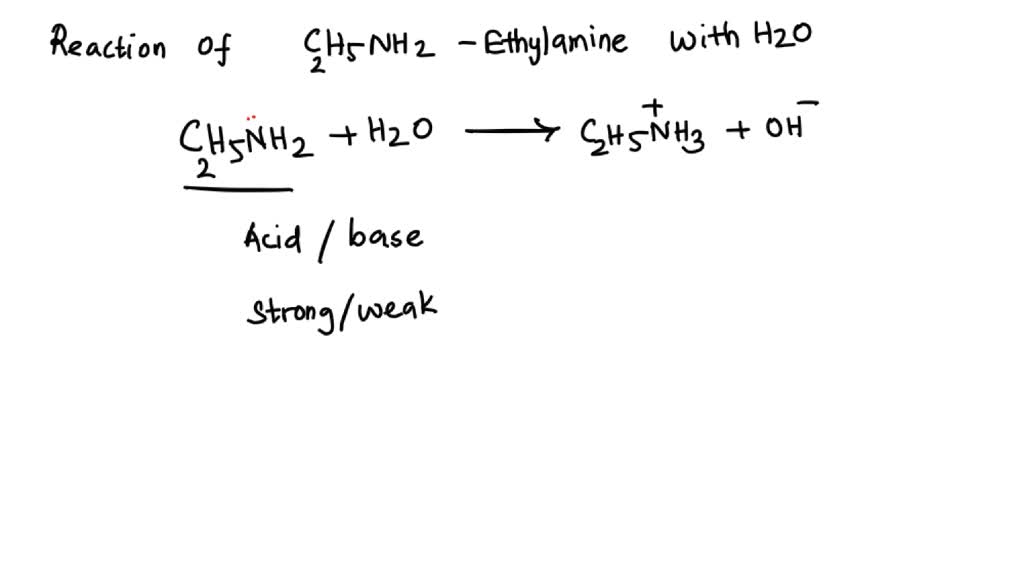
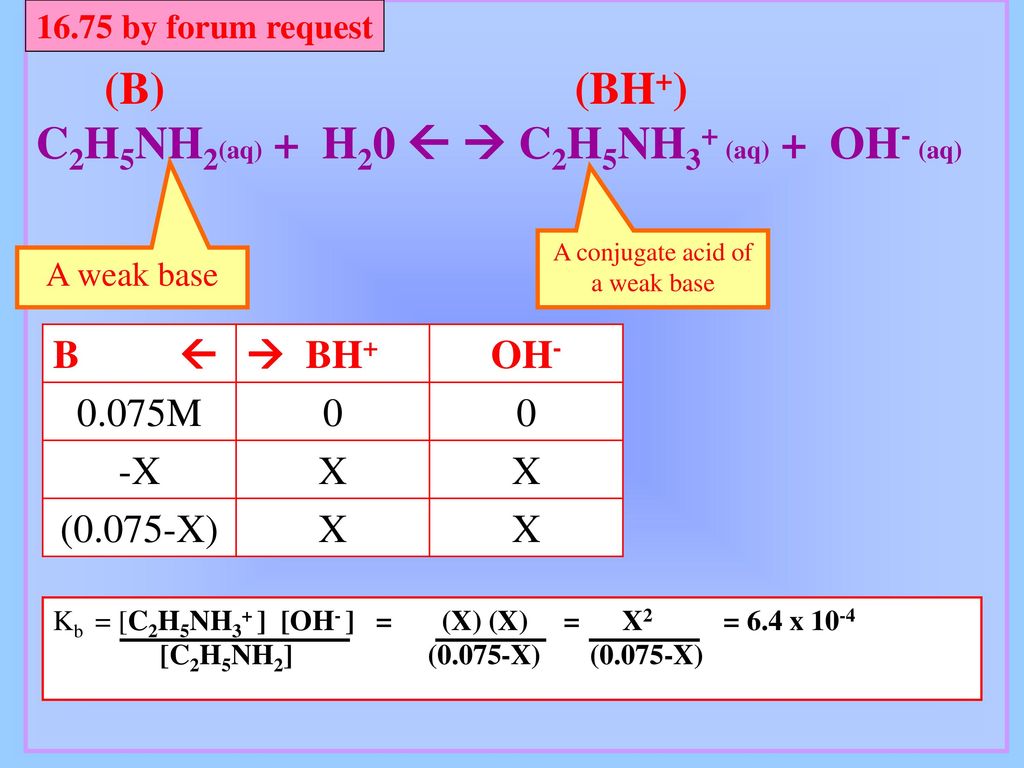
SOLVED: 5. When added to water, ethylamine undergoes the following reaction: C2H5NH2 + H2O C2H5NH3+ + OH- Is ethylamine an acid or a base in this reaction? Strong or weak? How do
![SOLVED: The base-dissociation constant of ethylamine (C2H5NH2) is 6.4 × 10-4 at 25.0 °C. The [H+] in a 1.6 × 10-2 M solution of ethylamine is M. SOLVED: The base-dissociation constant of ethylamine (C2H5NH2) is 6.4 × 10-4 at 25.0 °C. The [H+] in a 1.6 × 10-2 M solution of ethylamine is M.](https://cdn.numerade.com/project-universal/previews/776905ac-1583-4182-adc4-a5a01e13e224.gif)
SOLVED: The base-dissociation constant of ethylamine (C2H5NH2) is 6.4 × 10-4 at 25.0 °C. The [H+] in a 1.6 × 10-2 M solution of ethylamine is M.
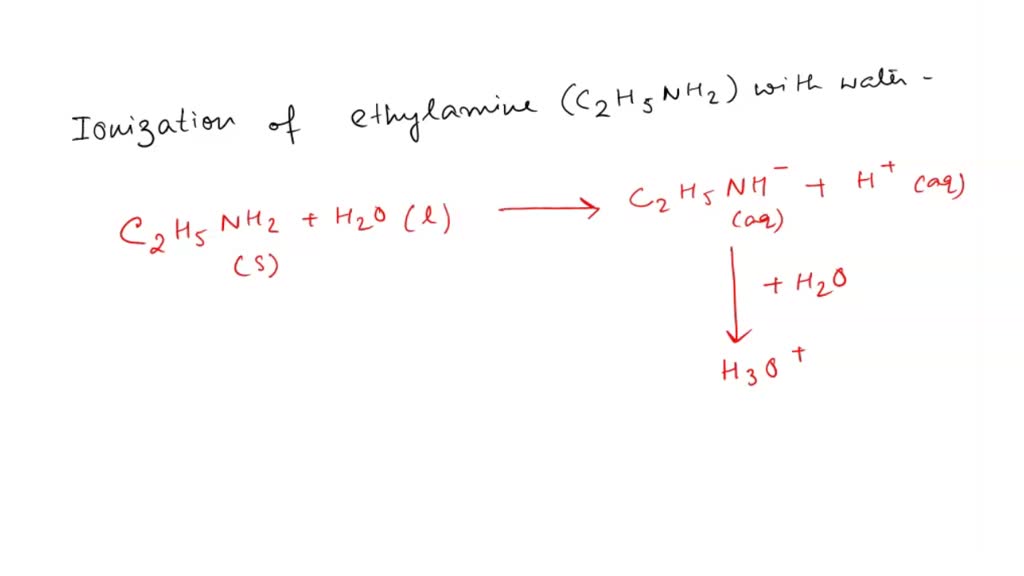
SOLVED: Write the equation for the ionization of ethylamine (C2H5NH2), a weak molecular base, with water