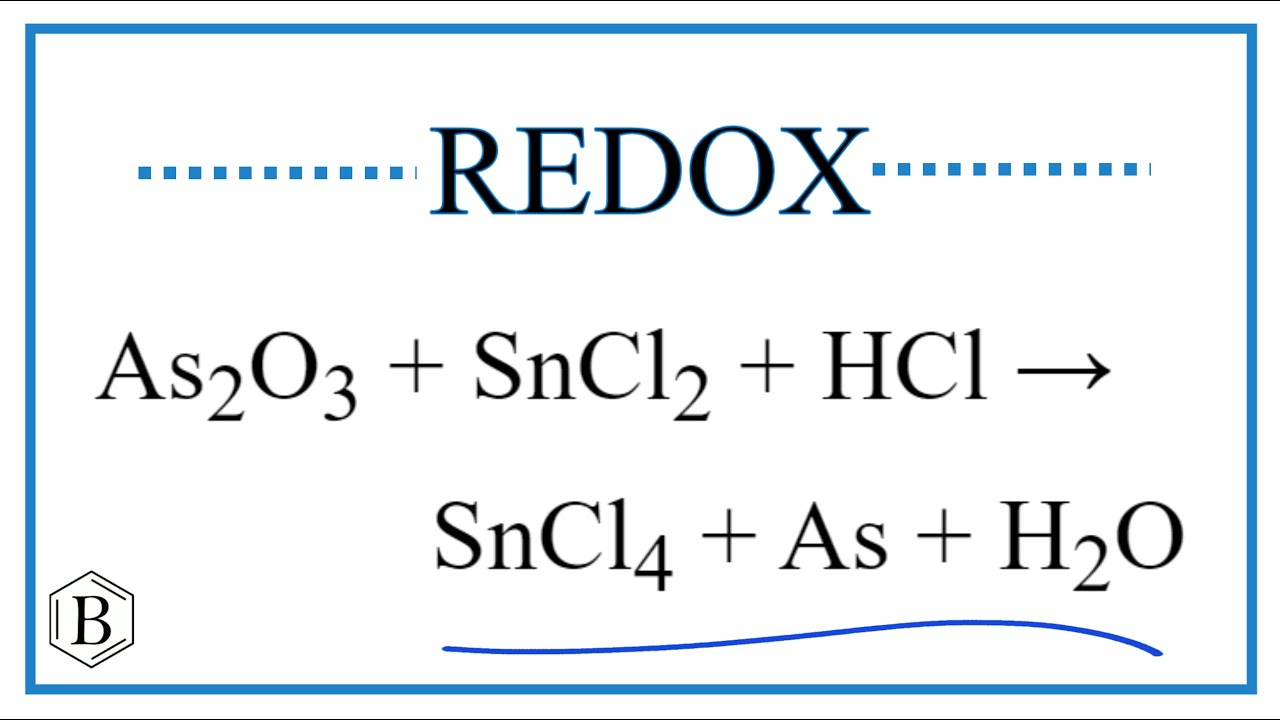
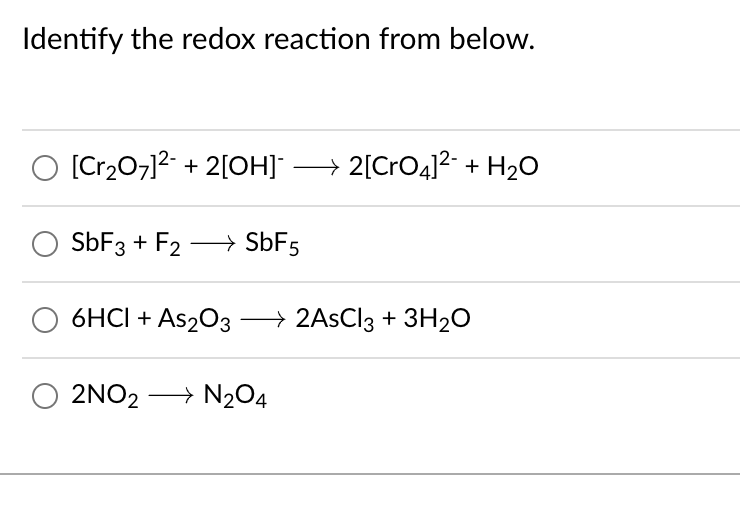SOLVED: Balance the following redox equation in acidic solution As2O3 (s) + NO3-(aq) –> H3AsO4 (aq) + N2O3 (aq) What is the coefficient in front of As2O3 in the balanced equation? P.S.
Balance the following equation using oxidation number method AS2S3 + HNO3 + H2O → H3ASO4 + H2SO4 + NO - Sarthaks eConnect | Largest Online Education Community
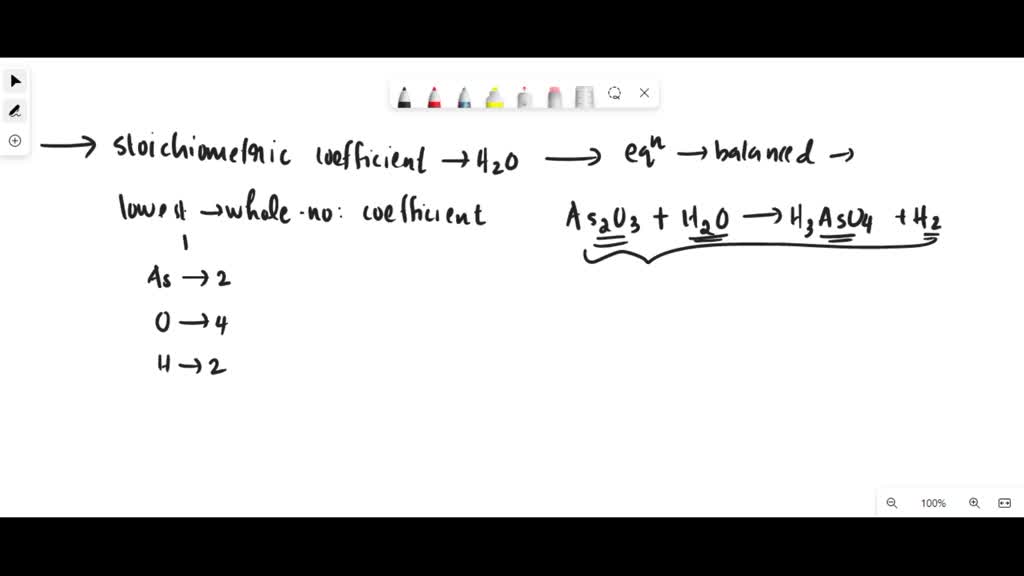
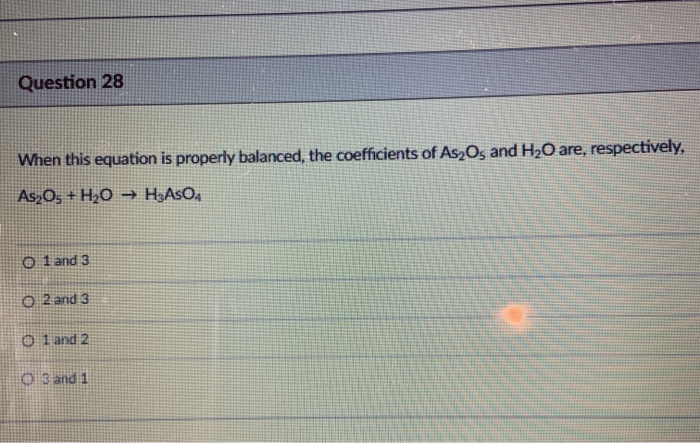
SOLVED: What is the stoichiometric coefficient for H2O when the following equation is balanced using the lowest, whole-number coefficients? As2O3 + H2O → H3AsO4 + H2
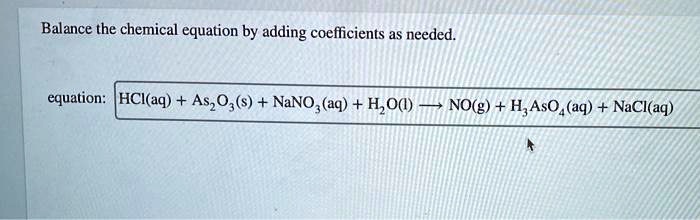
SOLVED: Balance the chemical equation by adding coefficients as needed. equation: HCl(aq) + As2O3(s) + NaNO3(aq) + H2O(l) â†' NO(g) + H3AsO4(aq) + NaCl(aq)
24. In the following reaction(unbalanced) n factor of As2S3 is As2S3 + H+ + NO3 1 NO + H2O + AsO4 3 + S04 2 (2) 4 (3) 24 (4) 28

SOLVED: 7-12. Arsenic(III) oxide (As2O3) is available in pure form and is a useful (but carcinogenic) primary standard for oxidizing agents such as MnO4. The As2O3 is dissolved in base and then
Which of the following reactions involves disproportionation reaction 2H2SO4+Cu—CuSO4+2H2O+SO2 As2O3+3H2S—As2S3+3H2O 2KOH+Cl2—KCl+KOCl+H2O Ca3P2+6H2O—Ca(OH)2+2PH3

SOLVED: A solution of iodine was standardized by titration with H3AsO3 prepared from weighing and dissolving 141.6 mg of pure As2O3. The titration reaction is H3AsO3 + I2 + H2O = H3AsO4 +
Może ktoś rozwiązać redoxa, robiąc to bilansem elektronowo-jonowym?As2O3 + HNO3 + H2O -> H3AsO4 + N2O3 - Brainly.pl